Disclaimer: Due to unforeseen difficulties, we have had to take down the images on this notes page. They will be replaced shortly. We apologise for the inconvenience, but hope that the new images will provide you with an even better learning experience.
- Place in order of reactivity: potassium, sodium, calcium, magnesium, zinc, iron, hydrogen and copper, by reference to the reactions, if any, of the elements with:
• water or steam
• dilute hydrochloric acid (except for alkali metals).
We can put metals in order of their reactivity by investigating how well they react with water or hydrochloric acid.
Reaction of metals and hydrogen with water or steam:
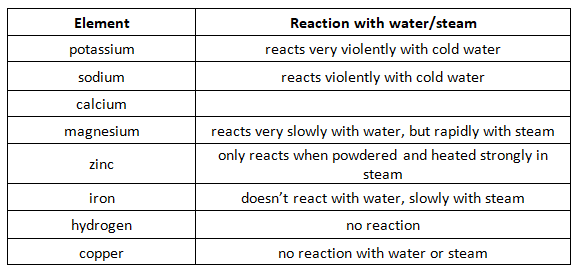
Reaction of metals and hydrogen with dilute (hydrochloric) acid:

We can see that the the above elements are put in their order of reactivity- the more reactive on the top (potassium, sodium, calcium etc) and the least reactive on the bottom (iron, hydrogen, copper). Only metals above hydrogen will react with water/steam and hydrochloric acid.
This is the reactivity series, from most reactive to least reactive (and a mnemonic to remember it!) :
- Compare the reactivity series to the tendency of a metal to form its positive ion, illustrated by its reaction, if any, with:
• the aqueous ions of other listed metals
• the oxides of the other listed metals.
Previosuly, we saw that a more reactive halogen will replace a less reactive halogen in a metal halide. We can see this is a competition to see which halogen combines best with the metal. We call this type of a reaction a displacement reaction. Metals can also ‘compete’ in a similar way.
Iron(III) oxide + aluminium ———-> iron + aluminium oxide
In the above, reaction the aluminium displaced the iron (think of it as ‘the aluminium stole the oxide and the iron was left alone’). This happened because aluminium is higher in the reactivity series than iron i.e. aluminium is more reactive than iron.
Let’s take another example, with an aqueous solution, and see how the metal ions form:
zinc + Copper(II)sulfate ———–> zinc sulfate + copper
Zn(s) + CoSO4(aq) ———————> ZnSO4(aq) + Cu(s)
Here, the zinc, being the more reactive metal, has clearly displaced copper to form zinc sulfate
Let’s break it up into half equations:
Zn(s) – 2e- ———–> Zn2+(aq) the zinc oxidised (lost electrons)
Cu2+(aq) + 2e- ———–> Cu(s) the copper reduced (gained electrons)
We can see that the more reactive metal, zinc, has lost electrons and the less reactive metal, copper, has gained electrons.
The more reactive a metal is, the more easily it loses its valence electrons.
It is easier to lose valence electrons and thus be more reactive, if:
- the valence electrons are further away from the nucleus. When they are farther away, the attraction of these electrons (negative charge) to the protons (positive charge)in the nucleus will be reduced. So it is easier to ‘let go’ of the electrons.
- there are more electron shells between the nucleus and the valence electrons. These shells shield the valence electrons from the charge of the nucleus (protons).
- there are fewer protons in the nucleus (less charge) to pull the electrons towards them
Zinc is more reactive than copper because its valence electrons are farther away from the nucleus and it has more shells. Zinc has more protons than copper but the other two conditions outweigh this one. So overall, zinc finds it easier to lose its valence electrons than copper, and so is more reactive, and thus can displace copper.
- Deduce an order of reactivity from a given set of experimental results.
When displacement reaction equations is given, you should be able to put them in order of reactivity. Just remember that the metal that displaces the other one is the more reactive one.
Notes submitted by Lintha
Click here to go to the next topic.
Click here to go to the previous topic.
Click here to go back to the Science menu.
life saver
LikeLike
i love your website
LikeLike
Thankyou!! Hope it’ll be able to get you to change that username soon! 😉
LikeLike
Very good
LikeLiked by 1 person