Disclaimer: Due to unforeseen difficulties, we have had to take down the images on this notes page. They will be replaced shortly. We apologise for the inconvenience, but hope that the new images will provide you with an even better learning experience.
- Identify and draw the structures of methane, ethane, ethene and ethanol.
(Don’t panic. You’ll understand these figures in more detail at the end of this topic!)
Methane – CH4 :
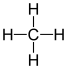
Ethane – C2H6 :
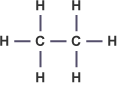 Ethene – C2H4 :
Ethene – C2H4 :
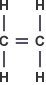 Ethanol – C2H5OH :
Ethanol – C2H5OH :
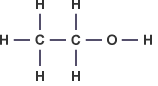
- Describe the concept of homologous series of alkanes and alkenes as families of compounds with similar properties.
Hydrocarbons are known as organic compounds, and they’re classified into homologous groups. Examples of homologous groups are alkanes and alkenes. Compounds in each homologous series have
- the same chemical reactions
- the same functional group (Eg. –OH, ‐COOH)
- the same general formula examples- Alkanes: CnH(2n+2) , Alkenes: CnH2n)
- similar, albeit slightly different, physical properties
- State the type of compound present, given a chemical name ending in -ane, -ene and -ol, or a molecular structure.
Alkanes are compounds that only contain hydrogen and carbon and possess no double bonds. They are saturated (by having only carbon single bonds, they have bonded with the maximum number of atoms). Their general formula is CnH(2n+2) . Examples are methane(CH4), ethane (C2H6), propane (C3H8) and butane (C4H10).
(Notice how the carbon atoms start at one and increase by one with each alkane. Their formulae also follow the general alkane formula).
Alkenes are compounds that only contain hydrogen and carbon but do possess one or more double bonds. They are unsaturated (by possessing one or more carbon double bonds, they have not bonded with the maximum number of atoms). Their general formula is CnH2n . Examples are ethene (C2H4), propene (C3H6) and butene (C4H8).
(Notice how the carbon atoms start from 2 and increase by one with each alkene. They too follow the general alkene formula).
Alcohols are compounds that contain hydrogen and carbon but also possess one hydroxyl group (-OH). Their general formula is CnH(2n+1)OH. Examples are methanol (CH3OH), ethanol (C2H5OH) and propanol (C3H7OH).
(The carbon atoms start from 1 and increase by one with each alcohol. They too follow the general alcohol formula).
- Name, identify and draw the structures of the unbranched alkanes and alkenes (not cis-trans), containing up to four carbon atoms per molecule.
(Tip:
‘meth’ – 1 carbon atom
‘eth’ – 2 carbon atoms
‘prop’ – 3 carbon atoms
‘but’ – 4 carbon atoms)
Notes submitted by Lintha
Click here to go to the next topic.
Click here to go to the previous topic.
Click here to go back to the Science menu.
Scoop di poop?
LikeLike
Hi, your image for propene is incorrect, it has 7 hydrogens but should have 6. The formula is correct with C3H6, just not the molecule shown, which shows the second carbon as bonded with 5 hydrogens.
LikeLike
Thanks for pointing it out! We’ve fixed it. Sorry for the error, we’re still proof-reading these notes!
LikeLike
Thank you so much, this site has been an exam saver!
LikeLike
Glad to hear it! Best of luck for all your exams!!
LikeLike
Hi, this is a very good website and I it has been very helpful to the preparation of my IGCSE’s. Thank you very much.
P.S The structural formula of Methane is wrong. It has 2 carbons and 6 Hydrogen molecules, which is Ethane.
LikeLike
In 14.2.1 only.
LikeLike
Hi Pedro!
Thanks for pointing that out!! We’re all on a bit of tight schedule, so we tend to make small mistakes here and there, and it’s really helpful when you help us find them 🙂
I’m glad the site’s helped you!
Good luck with your exams!
LikeLike